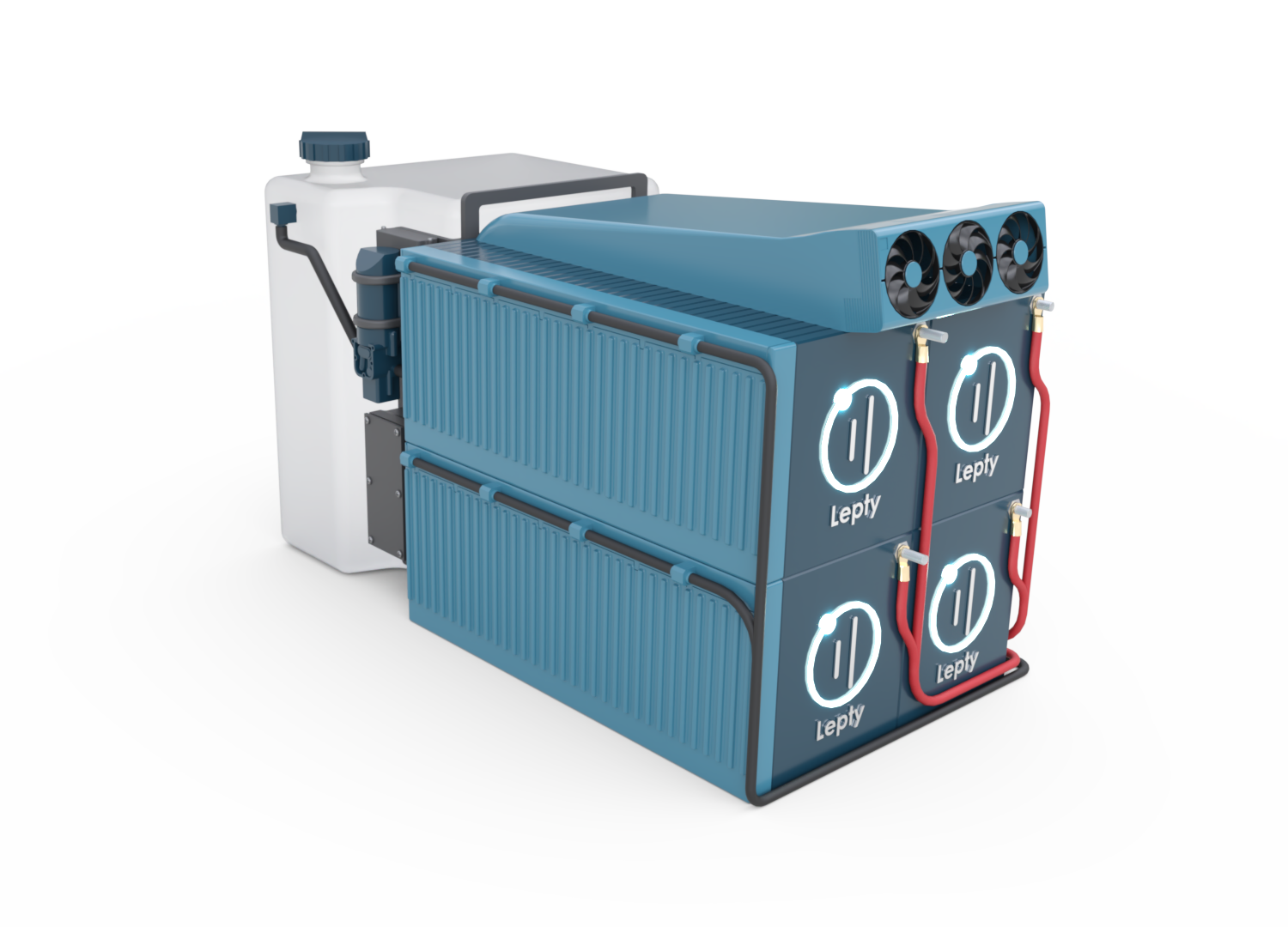
Clean, safe and efficient
Our generator uses aluminum as fuel to produce electricity.
The principle is simple: aluminum oxidizes with oxygen from the air and releases electrons — this flow of electrons creates an electric current.
No combustion, no exhaust gases: only a clean and controlled reaction that produces energy quietly and reliably.
Thanks to our unique process using fluid aluminum, our generator can be easily refueled: at an aluminum station or with a simple jerrycan, just like filling up with gasoline.

Clean
Emission-free and recyclable

Durable
Critical-metal-free catalysts

Light and compact
7x more compact than a Lithium battery of the same capacity

Water-based chemistry
Non-flammable, non-explosive

Fast refueling
Via aluminum station or jerrycan like gasoline
Clean, safe and efficient

100% recyclable
- About 2x more compact than a comparable Li-ion system
Water-based chemistry
Exceptional performance cathodes
At Lepty, we design and manufacture our own cathodes. The result of several years of research conducted at the University of Bordeaux, our technology stands out by eliminating costly traditional catalysts like platinum and by achieving extremely high power density.

At Lepty, we design and manufacture our own cathodes. The result of several years of research conducted at the University of Bordeaux, our technology stands out by eliminating costly traditional catalysts like platinum and by achieving extremely high power density.


Integrated know-how
At the heart of the Lepty generator lies aluminum-air technology, an innovative system based on the precise interaction of three elements: anode, cathode and electrolyte. Because performance depends on a constant search for balance, we at Lepty have chosen to maintain integrated expertise across all these subsystems.
And since our mission is to offer a solution that can be integrated by other manufacturers, we also develop mechanical solutions around the very core of our generator.
Anode manufacturing
To explore the full potential of aluminum, we develop and manufacture our own anodes. Lepty thus has specific knowledge of the properties of aluminum alloys that can be used as fuel.

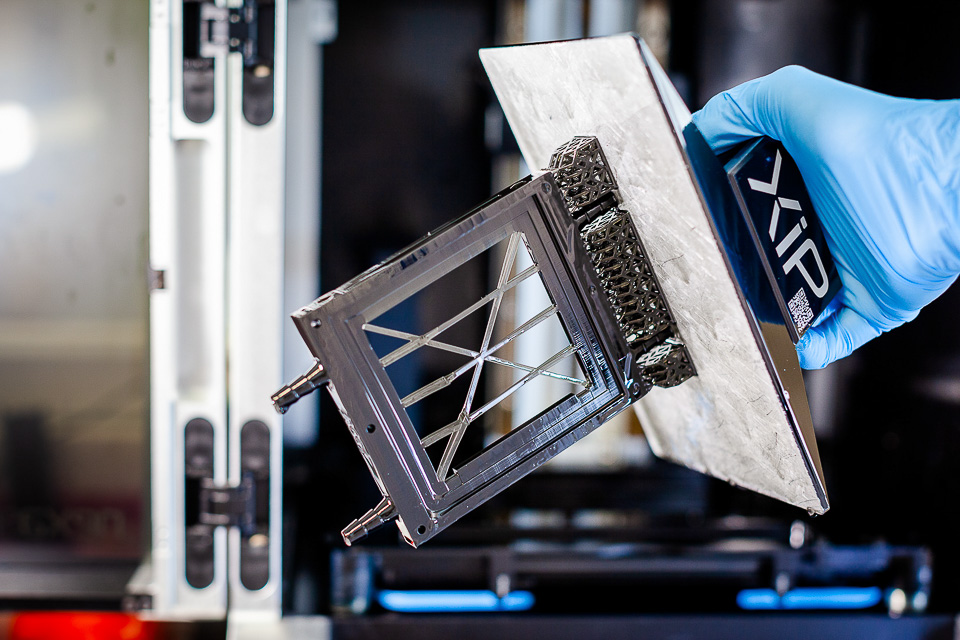
Mechanical assembly
New electrochemistry calls for innovative mechanical implementation. Thanks to 3D printing, we can perform rapid prototyping to test and evaluate our solutions without relying on long and expensive external processes.
Electrolyte formulation
The seat of many chemical interactions, the electrolyte is the fundamental component that links the anode and cathode. Finding its ideal composition is a complex process that must be conducted in harmony with the development of other components.
