Aluminum: the most energy-dense fuel
Aluminum is not only a modern material used in aerospace. It is also an exceptional fuel.
When it oxidizes, it releases a huge amount of energy. With an energy density of 22 kWh per liter, aluminum can contain 2.2 times more energy than gasoline.
In other words: aluminum is the most energy-dense fuel.
Hydrogen (700 bar)
Wood
Coal
Gasoline
Aluminum
Aluminum: the most energy-dense fuel
The ideal material for a secure and fair energy strategy
Aluminum is the most abundant metal in the Earth's crust, ahead of iron, and is available in many countries. Easy to handle, producible using low-carbon energy, and infinitely recyclable, it is an outstanding material to support Europe’s energy sovereignty.
European and circular
Easy to handle
Abundant and available
Low carbon impact
A virtuous zero-waste cycle
Aluminum is used as an energy carrier: part of the electricity consumed during its production is chemically stored in the material. Once loaded into the Lepty generator, the aluminum is available to react with air oxygen and generate electric current on demand.
Used aluminum becomes a metallic oxide called alumina, which is collected in a tank containing a water-based solution. When full, this tank is emptied and the liquid is collected by tanker trucks.
Since alumina is the raw material for producing aluminum, it is returned to existing plants to reconvert it into new aluminum.
Thus, the Lepty solution makes aluminum the first 100% recyclable fuel.


A technology that breathes
At the heart of the Lepty solution lies an innovative technology: the metal-air battery. This system combines an aluminum anode with an air-permeable cathode, separated by an electrolyte. The oxygen in the air, as it enters the cell, triggers electrochemical reactions that generate electricity.
Thanks to the direct use of ambient air, the Lepty generator is more compact and more efficient than any traditional battery.
For years, harnessing aluminum's energy potential was a challenge due to the lack of durability and stability. Lepty, a pioneer in aluminum-air technology, has developed unique know-how that finally makes this solution viable and relevant.
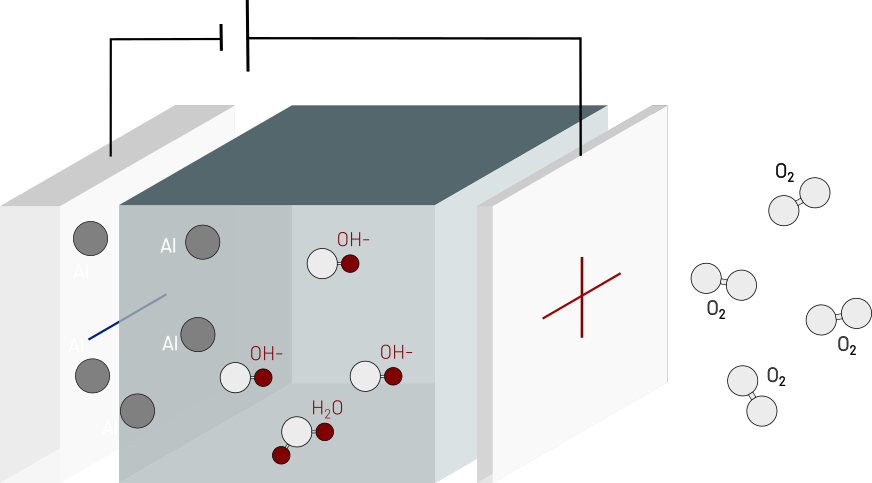
Electrochemistry in detail
The cell hosts two redox half-reactions, which together generate the electric current:
At the anode, aluminum oxidizes by consuming hydroxide ions (OH-).
At the cathode, oxygen and water react to form new hydroxide ions.
Hydroxide ions flow through the electrolyte from the cathode to the anode, while electrons leave the cell at the anode, travel through the load, and return to the system at the cathode.
Anode reaction
Al +3OH- → Al(OH)3 + 3e- E = -2.31 V (vs. SHE)
Cathode reaction
02 + 2H20 + 4e- → 4OH- E = +0.40V (vs. SHE)
Overall cell reaction
4Al + 3O2 + 6H2O→ 4Al(OH)3 E = -2.71 V